By Kyle Gaudet
Advances in Medical Devices
Americans today have a much higher life expectancy than before significant medical instrumentation was being invented. Over the past 25 years there has been a rapid increase in technology associated with medical instruments. This accelerated increase is due to the expansion of scientific and engineering knowledge.
This expansion comes from bringing clinical and biomedical research, mathematics, engineering, and physical sciences together. Most of the research has taken place inside university laboratories as well as specialized government facilities. The creation of new medical devices has developed into one of the largest industries in the world. There are many benefits associated with revolutionary implantable devices and how they may affect patients.
These devices are now used in conjunction with almost every organ. Improved function of the human body is one of the many positive factors. In many cases however, these procedures can come with risks or complications. Any time the human body is opened during a procedure, infections may arise. It is important to avoid these risks at all costs.
Role of Small and Large Firms in the Industry
There are a number of pioneering firms that invent and produce medical instruments. About half of the medical device industry is represented by small firms. These small companies play a crucial role in the development of new medical devices. Typically, they are very quick to adapt, identify, and target market niches. These small companies also have high demand for funding to keep up with the ever-changing technology.
Small firms, as well as philanthropic individuals, contribute greatly to most of the innovations of new medical devices. Larger firms play the role of testing these new innovations and mastering the most efficient production of the devices. In terms of sales, large companies easily lead this category. They have many more representatives and employees that can promote and sell their products. This brings greater profits as well as expenditures.
Some devices demand greater capital costs requiring larger-scale investments. These larger investments can potentially limit smaller competitors from entering the market.
Regulatory Framework: Medical Device Amendments of 1976
In 1976, the Medical Device Amendments were created which required all new devices introduced into the market were shown to be safe and effective through repeated testing. These amendments divided medical instruments into three categories based on the potential risks to the patient.
The first category is known as Class I. These devices could include bandages, hand-held instruments, or tongue depressors. This category contains the lowest potential risk to patients. This class makes up about 30% of all medical devices.
Class II is the second category. This class makes up around 60% of instruments. Class II instruments involve immediate risk devices and include X-ray devices, CT scanners, and infusion pumps. Class II application procedures involve more invasive techniques that, if not conducted properly, could lead to problems. Class II devices all need to undergo a 501k review to determine if the device is essentially equivalent to an existing instrument.
The last category are the Class III instruments. The most notable difference between this class and the first two is these instruments are life supporting. Without these devices, patients would experience impairment to their health and daily living. This category is meant to sustain the life to a sick patient. Because these devices are used in the most serious cases, they have the potential for causing injury or illness.
Examples would include deep brain stimulators, pacemakers, or laser angioplasty devices. Only about 10% of all medical devices are categorized into this group. All manufacturers need to submit a pre-market approval application to allow these instruments on the market. A series of clinical studies are required for Class III instrument approval.
Types of Medical Implants
Medical implants are placed inside or outside of the body. These implants are designed to replace or improve the function of various organs. There are also implants designed to deliver medication, support organs or tissues, and monitor body functions. Naturally occurring implants can be made from bone grafts, skin, or other tissues. Artificial implants are usually made from plastic, metal, or ceramic. These devices can be placed permanently or could be removed over time once the body heals.
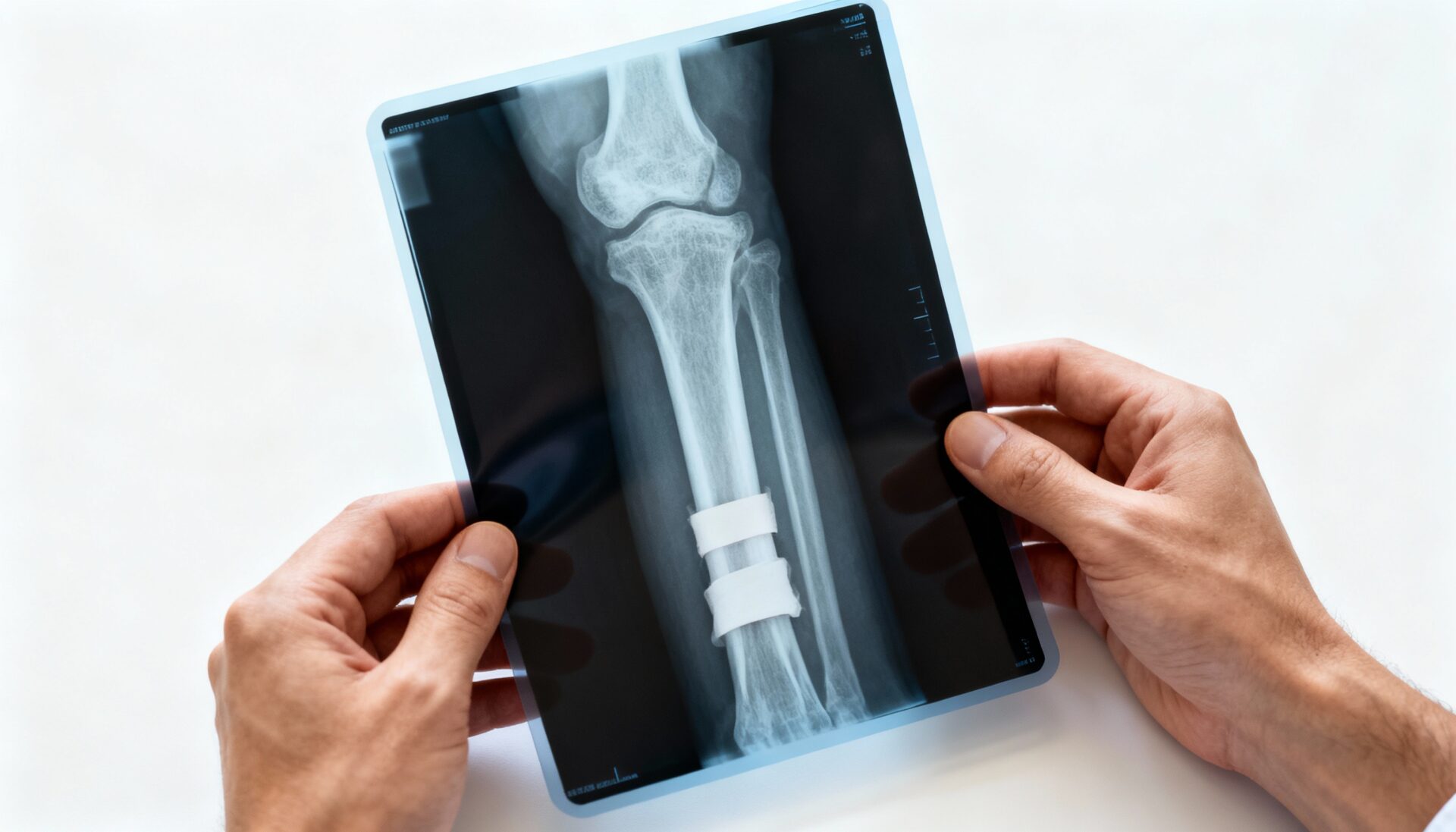
Some examples include screws, rods, or plates used to fix broken bones. After the bone is fully healed, the implants may be removed when they are no longer needed. An artificial knee replacement to cure arthritis is an example of a permanent implant. Implants can be used when a tragic injury occurs but can also be beneficial when joints are subjected to heavy use and are simply worn out.
Joint replacements are commonly used all over the world. These implants often create a massive change in overall quality of life for impaired patients. Although the procedures are quite extensive, the results are usually welcomed and appreciated.
Risks and Complications of Implants
While there are many benefits associated with implants, risks always accompany opening the body. Should a patient receive a soiled or unsterile implant, infections can occur. Such infections could lead to serious sickness or possible amputation. Implant failures can also occur. They may be due to adverse reactions to the materials in the implant or improper insertion by the physician. These risks can arise during placement or removal and may be immediate or a delayed reaction.
Some of the more subtle problems include bruising, pain, swelling, and redness. These reactions are normal to see after insertion or removal. Infections are also common with the introduction of implants. These infections are often a result of contamination at the time of surgery. If this occurs, draining may be necessary near the insertion area, or medication could also be used to start the healing process. If these steps do not work, the implant might have to be removed to minimize further complications.
Long-Term Issues with Implants
Over time medical implants could break, move, or completely stop working. These could cause serious problems depending upon the type of implant used. If a pacemaker were to stop working in a patient with an irregular heartbeat, they might not get the necessary blood flow to the body. If a knee replacement were to shift after a hard fall, there would also be complications. The physician would need to reopen the implant site to replace or repair the implant.
Conclusion
Medical devices have greatly improved the options available to healthcare professionals. Without advances in medical instruments and implantation, many procedures would be difficult if not impossible. These new technologies have provided patients relief from pain and suffering and an improved Quality of Life.
Healthcare workers are being given more tools to help take positive steps towards recovery and wellness. Although complications may arise through these procedures, greater benefits are often the ultimate outcome.
References
Center for Devices and Radiological Health. “Implants and Prosthetics.” U.S. Food and Drug Administration, FDA, www.fda.gov/medical-devices/products-and-medical-procedures/implants-and-prosthetics.
Gelijns, Annetine C. “3. The Development of Medical Devices.” Technological Innovation:Comparing Development of Drugs, Devices, and Procedures in Medicine., U.S. NationalLibrary of Medicine, 1 Jan. 1989, www.ncbi.nlm.nih.gov/books/NBK222708/.
“Medical Implant.” Medical Implant – an Overview | ScienceDirect Topics, www.sciencedirect.com/topics/medicine-and-dentistry/medical-implant.
Jill Jin, MD. “FDA Authorization of Medical Devices.” JAMA, JAMA Network, 22 Jan. 201jamanetwork.com/journals/jama/fullarticle/1817798.
Joung, Yeun-Ho. “Development of Implantable Medical Devices: from an Engineering Perspective.” International
Neurourology Journal, Korean Continence Society, Sept. 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3797898/.